Mechanisms and Mitigation of Radiation Damage in X-ray Crystallography
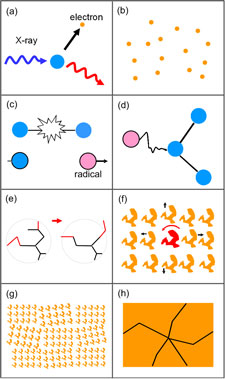
Radiation damage by the illuminating X-rays limits the amount of structural information that can be obtained from protein and virus crystals, and the quality and accuracy of the resulting molecular structures. Advances in synchrotron sources and detectors have made it possible to collect diffraction data from crystals as small as a few microns, but here the constraints of radiation damage are especially severe.
Resolution and dose dependence of radiation damage to biomolecular systems.
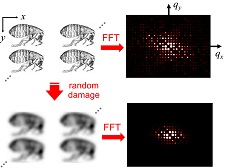
What is the underlying relation between diffracted intensity, resolution, and dose? Motivated by our work using X-ray microbeams, we re-analyzed data from previous radiation damage studies to account for spatially nonuniform irradiation during data collection. We found that the local intensity-dose relation at T=100 K is strictly exponential, and that the decay constant varies roughly as the square of the resolution (in Angstroms). We then analyzed perhaps the simplest physics-based model for radiation damage, and showed that it reproduces both of these experimental observations. These results suggest that radiation damage may introduce substantial errors in measured structure factors, and that more sophisticated corrections for radiation damage must be added to crystallographic analysis software. They also show that models used to fit radiation damage for the last 50 years are invalid.
We have also shown that the maximum tolerable dose for atomic-resolution (sub-2 Angstrom) data collection is approximately 10 MGy at T=100 K, roughly 1/3 the widely cited Garman limit, but consistent with the first measurement of dose limits by Teng and Moffat and with Henderson's estimate.
Resolution and dose dependence of radiation damage in biomolecular systems. H. Atakisi, L. Conger, D. W. Moreau, and R. E. Thorne. IUCrJ 6, 1040-1053 (2019).
Spatiotemporal response of protein crystals to intense X-ray microbeams
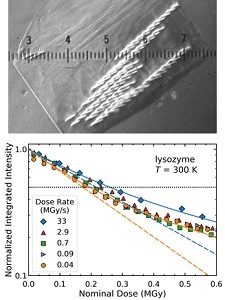
Serial synchrotron-based crystallography using intense microfocused X-ray beams, fast-framing detectors and protein microcrystals held at 300 K promises to expand the range of accessible structural targets and to increase overall structure-pipeline throughputs. We examined the time-, dose- and temperature-dependent evolution of crystal diffraction at dose rates approaching 50 MGy/s. At all temperatures and dose rates, the integrated diffraction intensity for a fixed crystal orientation shows nonexponential decays with dose. These arise because of nonuniform crystal illumination and damage by the microbeam, and the resulting "hole-burning"-like spatial evolution of diffracted intensity within the illuminated crystal volume. To quantify radiation-damage lifetimes and the damage state of diffracting crystal regions, we defined revised diffraction-weighted dose (DWD) showed that for Gaussian beams the DWD becomes nearly independent of actual dose at large doses.
Lifetimes and spatio-temporal response of protein crystals in intense X-ray microbeams. M. A. Warkentin, H. Atakisi, J. B. Hopkins, D. Walko, and R. E. Thorne, IUCrJ 4, 785-794 (2017).
Dose dependence of damage at T = 300 and 100 K
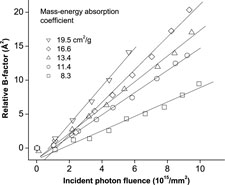
We have shown that radiation damage to protein crystals is a strict function of dose (energy absorbed/kg), and is independent of crystal composition.
At T=100 K, all protein crystals examined to date exhibit approximately equal global radiation sensitivities (damage/dose), regardless of sequence, structure, solvent content and heavy (large Z) atom content. At T=300 K, sensitivities are typically 20-50 times larger than at T=100 K, but some crystals with high solvent contents can be ~1000 times more sensitive.
Quantifying X-ray radiation damage in protein crystals at cryogenic temperatures. J. Kmetko, N. S. Husseini, M. Naides, Y. Kalinin and R. E. Thorne, Acta Cryst. D 62, 1030-1038 (2006).
Can radiation damage to protein crystals be reduced using small molecule compounds? J. Kmetko, M. Warkentin, U. Englich and R. E. Thorne, Acta Cryst. D 67, 881-893 (2011).
Temperature dependence of radiation damage
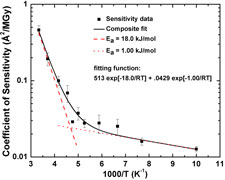
We have measured the full temperature dependence of global radiation damage at temperatures between 300 and 100 K. Two distinct damage regimes are observed, that can be fit using Arrhenius functions: a high temperature regime with an activation energy of 18 kJ/mol, and a low temperature regime with an activation energy of 1 kJ/mol. The crossover occurs near the protein-solvent glass transition at ~200 K. Above the transition, damage is dominated by diffusive motions of solvent, radicals, side chains and larger structural units. Below the transition these motions freeze out and damage may be vibrationally assisted.
Glass transition in thaumatin crystals revealed through temperature dependent radiation sensitivity measurements. M. Warkentin and R. E. Thorne, Acta Cryst. D 66, 1092-1100 (2010).
Effectiveness of free radical scavengers
Small-molecule free-radical scavengers can reduce radiation damage to proteins in solution and in cells, and there have been many reports of their effectiveness in protecting protein crystals. We have shown that for protein crystals at T=100 K, none of 19 small molecule compounds has any effect on global damage. At 300 K, only sodium nitrate appears to have a (small) beneficial effect, and several known scavengers actually increase damage. Scavengers are ineffective in protecting protein crystals from global damage because a large fraction of the incident radiation is absorbed by protein atoms and because the ratio of scavenger molecules to protein molecules is too small to provide appreciable competitive protection.
Can radiation damage to protein crystals be reduced using small molecule compounds? J. Kmetko, M. Warkentin, U. Englich and R. E. Thorne, Acta Cryst. D 67, 881-893 (2011).
Spatial distribution of radiation damage
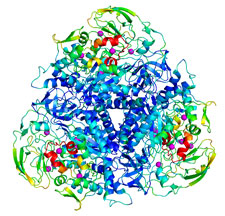
We have measured the spatial distribution of radiation damage within the unit cell of thaumatin and urease crystals at temperatures from 25 K to 300 K. The nature of damage changes dramatically at approximately 180 K. Above that temperature, the role of solvent diffusion is apparent and solvent-exposed turns and loops are especially sensitive. In urease, a flap covering the active site is the most sensitive part of the molecule, and nearby loops show enhanced sensitivity. At all temperatures, the component of damage that is spatially uniform within the unit cell accounts for more than half the total increase in atomic B-factors, and may arise from lattice level rather than local disorder. The effects of primary structure on radiation sensitivity are small compared to those of tertiary structure, local packing, solvent accessibility, and crystal contacts.
Spatial distribution of radiation damage to crystalline proteins at 25-300 K. M. Warkentin, R. Badeau, J. B. Hopkins and R. E. Thorne, Acta Cryst. D68, 1108-1117 (2012).
Dark progression and radiation damage timescales
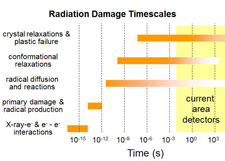
Radiation damage processes from initial X-ray photon absorption/scattering through to crystal lattice relaxation and cracking occur on an extremely wide range of timescales. Which of these processes dominate in causing fading of diffraction spots and loss of resolution in X-ray diffraction patterns - the manifestations of damage that are relevant in protein crystallography?
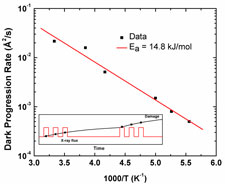
We observed and quantified the increase or progression of radiation damage that occurs after the X-ray beam is turned off. On timescales of 200 to 1200 s, no dark progression is observed below T=180 K or above T=240 K, and between these temperatures the rate of dark progression increases with temperature. Based upon these results, we were able to estimate timescales for the manifestation of damage in X-ray diffraction patterns at room temperature to be of order 1 second. This is roughly six orders of magnitude larger than the timescale for free-radical chemistry, indicating that structural relaxation processes that occur far downstream of radical chemistry dominate diffraction spot fading.
Dark progression reveals slow timescales for radiation damage between T=180 and 240 K. M. Warkentin, R. Badeau, J. Hopkins and R. E. Thorne, Acta Cryst. D 67, 792-803 (2011).
Outrunning radiation damage

If X-ray data is collected on a timescale short compare with that of a given damage process, that process should be outrun. X-ray free-electron laser experiments show that data collection in 10-13 s - using ultra-intense X-ray pulses - can outrun nearly all damage. Using X-ray flux densities available at current third-generation synchrotron sources, we have shown that data collection in ~1 s combined with modest cooling to T=260 K allows approximately half of damage to be outrun; at room temperature, recent experiments suggest that 75% of damage can be outrun by collecting data in 0.1 s. This should facilitate a significant expansion in room-temperature structural studies of proteins.
Global radiation damage at 300 and 260 K with dose rates approaching 1 MGy/s. M. Warkentin, R. Badeau, J. B. Hopkins, A. M. Mulichak, L. J. Keefe and R. E. Thorne, Acta Cryst. D68, 124-133 (2012).
Global radiation damage: Temperature dependence, time dependence, and how to outrun it. M. Warkentin, J. B. Hopkins, R. Badeau, A. M. Mulichak, L. J. Keefe and R. E. Thorne. J. Synch. Rad. 20, 7-13 (2013).
Lifetimes and spatio-temporal response of protein crystals in intense X-ray microbeams. M. A. Warkentin, H. Atakisi, J. B. Hopkins, D. Walko, and R. E. Thorne, IUCrJ 4, 785-794 (2017).