Research
Single-particle cryoelectron microscopy
Advances in direct electron detectors, microscope phase plates, and analysis software in the last decade have transformed single-particle cryo-electron microscopy (cryoEM) into a workhorse tool for determining near-atomic-resolution structures of large biomolecules and their complexes. We are focused on unraveling physical phenomena in sample preparation, cryocooling, and sample interaction with electron beams that limit achievable resolution and on developing methods for time-resolved cryo-EM of biomolecular function. (more)
Movies of Biomolecules in Action: Time-Resolved Crystallography
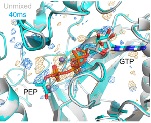
Making atomic-resolution movies of biomolecules (e.g., enzymes) in action has been a long-term goal in structural science. The most popular current methods, based on serial crystallography at X-ray free-electron laser or synchrotron sources, require 104 to 107 crystals per time point and complex apparatus operated by teams of researchers to execute each experiment. This has limited time resolved studies to a handful of well characterized systems. We have demonstrated an alternative approach that delivers millisecond time resolution that requires as few as 1 crystal per time point and remote data collection at a standard cryocrystallography beamline. Our approach promises to dramatically expand researcher access to time-resolved studies and the number of biomolecular targets suitable for such studies. (more)
New X-ray based approaches to probing protein structure and energy landscapes
The revolutions in molecular biology and molecular medicine are driven in part by the availability of high-resolution structures of proteins and other biological macromolecules. These structures provide insight into molecular function and a basis for rational approaches to the design of new medicines. However, nearly all such structures are determined at cryogenic temperatures, using either X-ray cryocrystallography or cryoelectron microscopy. Proteins under biological conditions exist in an ensemble of conformations that reflect their complex and multi-tiered energy landscapes, yet the overwhelming majority of X-ray structures are refined to a single conformation. We have been developing experimental methods that allow X-ray study of protein conformational ensembles in the presence of liquid water at temperatures down to 200 K, and for analyzing and interpreting this data. Small-angle X-ray scattering (SAXS) provides low resolution but high precision information about the structure of and interactions between biomolecules and their binding partners. We made the first successful demonstration of SAXS using cryocooled biomolecules, and are now working to develop cryoSAXS into a general purpose method that dramatically reduces sample volumes and data collection time per measurement and facilitates high-throughput screening of, e.g., potential pharmaceutical compounds. My company is collaborating with CHESS and Cornell researchers to develop improved methods for serial synchrotron crystallography. (more)
Solvent nanoconfinement, ice formation, and cryocooling in cryocrystallography
The overwhelming majority of biomolecular structures are determined using X-ray crystallography performed on crystals cooled to cryogenic temperatures. Since these crystals are typically between 40% and 80% solvent by volume, ice formation is a major problem, and even when ice does not form cooling degrades crystal order. We have characterized the disorder caused by cooling using a combination of X-ray imaging and diffraction methods. We have analyzed heat transfer from protein crystals, and developed methods for ultra-fast cooling (at >10,000 K/s), and also for obtaining ice-free diffraction at cryogenic temperatures from crystals cooled at only 0.1 K/s. We have shown that both freezing temperatures and ice nucleation rates of internal solvent are dramatically suppressed by the effects of nanoconfinement within the protein lattice. When internal ice forms, it is stacking disordered. We have measured the thermal contraction of aqueous solutions in which protein crystals are grown or soaked on cooling to cryogenic temperature, and shown that there is a large mismatch between this contraction and the observed contraction of the protein lattice. We have observed direct evidence that solvent flows driven by differential contraction are a major cause of cooling-induced disorder, and demonstrated methods by which this disorder can be reduced. We have improved an algorithm for detecting ice contamination in protein crystal structure factors, extended it to allow determination of the type of ice present (hexagonal as frost, near cubic or near hexagonal in surface solvent, stacking disordered inside) and thus diagnose its origin, and then analyzed nearly 100,000 unique entries in the Protein Data Bank (PDB) to determine the prevalence of different types of ice and how this has evolved over the last 20 years. (more)
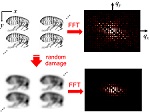
Radiation Damage in Biomolecular Crystallography and Biological Imaging
Radiation damage is a critical issue in all methods that use energetic particles (photons, electrons, neutrons) to probe the structure of biomolecular and biological systems, disrupting the structure and and limiting the amount of information that can be obtained from each sample. We have examined several aspects of radiation damage to protein crystals and to proteins in solution, and are developing improved approaches for mitigating and modeling this damage. (more)
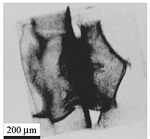
Disorder and structural transitions in protein crystals
We have used a combination of physical methods including X-ray imaging and diffraction and two-photon fluorescence imaging to characterize disorder in protein crystals and how this disorder arises during crystallization and post-crystallization crystal treatments. We have determined the elastic constant tensor of a protein crystal, and studied hydration-induced structural transformations. (more)
Contact line pinning and drop stabilization
We have examined the pinning and sliding of liquid drops on solid surfaces, developed a method for holding drops in place that is superior to conventional hydrophilic-hydrophobic patterning, and used this as the basis for a novel protein crystallization device. (more)
Physics of Cryopreservation
Cryopreservation is a key technology in modern biology and biomedical practice, used for long term storage of proteins (including protein-based medications), cells (egg, sperm, stem), tissues, and entire organs. We are exploring the (largely unexplored) physics of cryopreservation of small samples performed under conditions of rapid cooling and warming. We have examined ice nucleation and aqueous glass formation during rapid cooling of aqueous solutions, explained how cryoprotectants reduce ice nucleation, measured the densities of aqueous glasses at cryogenic temperatures, and examined ice formation in nominally vitrified samples during rapid warming. These results provide insight into the physics of aqueous glass formation at large cooling rates, and a rational basis for optimization of cryopreservation protocols. (more)
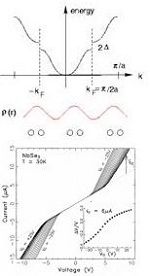
Charge Density Wave Conductors
Low-dimensional electronic materials that undergo transitions to charge or spin-density wave states are among the most remarkable conducting materials ever discovered. They exhibit extremely diverse phenomena having analogs in superconducting, magnetic, and pattern forming systems. We established the nature of CDW pinning by impurities, the critical role of finite size effects, and the absence of pinning by crystal surfaces; made the first successful demonstration of gating (field effect modulation) of nanowires of a quasi-one-dimensional material (inspiring the first gating of carbon nanotubes); characterized the static and sliding structure of the CDW state using synchrotron-based X-ray diffraction and imaging; elucidated the fundamental experimental phenomenology of both longitudinal CDW phase slip (essential for conversion between collective and single-particle current) and of shear phase slip, and determined the longitudinal and shear elastic constants of the CDW; established the role of thermal fluctuations in collective CDW transport; and established the detailed temperature- and electric-field-dependent "phase diagram" of CDW transport, including discovery of the most remarkable transport phenomenon exhibited by CDW/SDW materials: coherent collective creep. We also developed methods to prepare the highest quality crystals of three common CDW materials, and these were used in experiments by several groups around the world. (more)
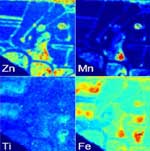
X-ray Imaging of Ancient Artifacts
In collaboration with CHESS staff scientists and Cornell Classics faculty, we used synchrotron-based X-ray fluorescence imaging as a tool for studying ancient artifacts, including its first application to ancient inscriptions on stone and to pre-Columbian Mesoamerican ceramics.
(more)