Disorder and Structural Transitions in Protein Crystals
Origin of disorder in protein crystals
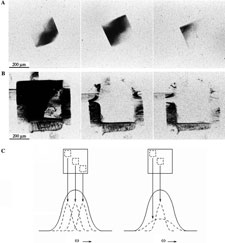
We have used a variety of X-ray diffraction techniques including X-ray topography and high resolution mosaicity and q scans to examine the nature and origin of disorder in protein crystals, and have analyzed the possible contributions of observed defects in degrading diffraction properties. Crystal mosaicity is dominated by micron and larger scale disorder associated with cracks and dislocations, but diffraction resolution is dominated by unit-cell-scale heterogeneity, such as that arising when proteins have flexible loop regions.
X-ray Topographic Studies of Protein Crystal Perfection and Growth I. Dobrianov, K. D. Finkelstein, S. G. Lemay, and R. E. Thorne. Acta Cryst. D 54, 922 (1998).
X-Ray Diffraction Studies of Protein Crystal Disorder. I. Dobrianov, C. Caylor, S. G. Lemay, K. D. Finkelstein, and R. E. Thorne, J. Cryst. Growth 196, 511 (1999).
Growth and disorder of macromolecular crystals: insights from atomic force microscopy and X-ray diffraction studies. A. J. Malkin and R. E. Thorne, Methods 34, 273-299 (2004).
Macromolecular impurities and disorder in protein crystals
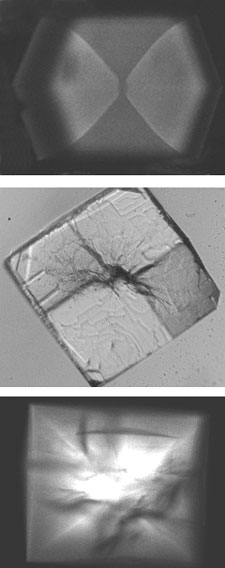
Unlike in small-molecule crystals growth, the solutions used in protein crystal growth are extremely impure, typically containing several percent by weight of other proteins, aggregated proteins and protein fragments. We have used a combination of X-ray topography, X-ray diffraction, and two-photon fluorescence imaging to examine how macromolecular impurities affect growth and order of protein crystals. Macromolecular impurities incorporate with different concentrations in different crystal growth sectors. They also incorporate in much larger concentrations in the crystal core. These concentration differences create stresses that drive crystal cracking and mosaicity broadening.
Two-Photon Fluorescence Imaging of Impurity Distributions in Protein Crystals. C. L. Caylor, I. Dobrianov, C. Kimmer, W. Zipfel, and R. E. Thorne, Phys. Rev. E 59, R3831 (1999).
Macromolecular Impurities and Disorder in Protein Crystals. C. L. Caylor, I. Dobrianov, S. G. Lemay, C. Kimmer, S. Kriminski, K. D. Finkelstein, W. Zipfel, B. R. Thomas, A. A. Chernov, and R. E. Thorne, Proteins: Structure, Function, and Genetics 36, 270 (1999).
Structural transitions in protein crystals
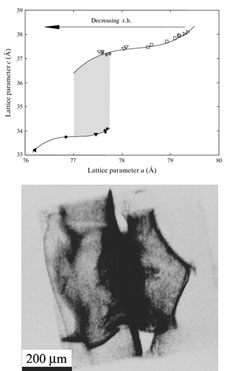
We have used X-ray diffraction imaging (X-ray topography) and other X-ray measurements to characterize the dynamic response of protein crystals to dehydration. Most protein crystals under first-order structural phase transitions at a well-defined humidities. Crystals can undergo significant dehydration and lattice shrinkage without loss of order, but large stresses that develop during phase transitions as the boundary between transformed and untransformed regions can severely disorder the crystal lattice. These dehydration-induced transitions serve as a model for transitions induced by changes in temperature and/or chemical environment.
Dynamic Response of Tetragonal Lysozyme Crystals to Changes in Relative Humidity: Implications for Post-Growth Crystal Treatments. I. Dobrianov, S. Kriminski, C. L. Caylor, S. G. Lemay, A. Kisselev, K. D. Finkelstein, and R. E. Thorne, Acta Cryst. D 57, 61 (2001).
Elastic properties of protein crystals

In collaboration with Tom Duffy and Sergio Speziale at Princeton University, we have used Brillouin scattering to make the first measurements of the elastic constant tensor of a protein crystal.
Measuring the Elastic Properties of Protein Crystals by Brillouin Scattering. C. L. Caylor, S. Speziale, S. Kriminski, T. Duffy, C-S Zha and R. E. Thorne, J. Cryst. Growth 232, 498 (2001).
Sound Velocity and Elasticity of Tetragonal Lysozyme Crystals by Brillouin Spectroscopy. S. Speziale, C. L. Caylor, S. Kriminski, C.-S. Zha, R. E. Thorne and T. S. Duffy, Biophys. J. 85, 3202-3213 (2003).