CryoSAXS for Determining Low-Resolution Protein Structures
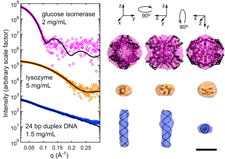
Small-angle X-ray scattering (SAXS) is a widely used technique for obtaining low-resolution structures of proteins, nucleic acids and complexes. SAXS experiments measure molecules in solution at room temperature, without the need for labeling or crystallization. However, radiation damage occurs extremely rapidly, and currently limits the application of SAXS to molecules that can be produced in microgram quantities. As in protein crystallography, cooling SAXS samples to T=100 K might be expected to dramatically reduce radiation damage and sample volume requirements. However, SAXS is exceptionally sensitive to formation of ice and other inhomogeneities.
Building upon our expertise in cryocooling, cryopreservation and aqueous glass formation, we have demonstrated the feasibility of performing SAXS on flash-cooled protein and nucleic acid samples. Cryocooled proteins and nucleic acids can withstand doses at least two and up to five orders of magnitude larger than room temperature samples. Accurate T=100 K particle envelope reconstructions can be obtained from sample volumes as small as 15 nL, a factor of 1000 smaller than in current practice. Cryo-SAXS will thus enable structure determination of difficult-to-express proteins and biologically important, highly radiation-sensitive proteins including light-activated switches and metalloenzymes. This project is a collaboration with the group of Prof. Lois Pollack in A&EP.
Breaking the radiation damage limit with cryo-SAXS. S. P. Meisburger, M. Warkentin, H. Chen, J. B. Hopkins, R. E. Gillilan, L. Pollack and R. E. Thorne. Biophys. J. 104, 227-236 (2013)